
Präklinische Theranostik
(Preclinical Theranostics)
Research
Our research focuses on theranostics with the goal to deliver new insights into tumor biology, heterogeneity and metabolism, and their relationship and relevance to functional imaging and biomarker-driven treatments in nuclear medicine.
The term theranostics combines the words diagnosis and therapy; it represents a precision medicine approach relying on a specific targeted diagnostic test that helps to select patients for a specific targeted therapy. In nuclear medicine, radioligands are used for theranostics. Radioligands are radio-labelled molecules that bind to a cell surface receptor on target cells. When coupled to radio-isotopes like Gallium-68 or Fluor-18, radioligands can be used for imaging with positron emission tomography (PET), single-photon emission computed tomography (SPECT), or scintigraphy. This imaging facilitates the selection of patients for subsequent radioligand therapy. Radioligand therapy is an emerging treatment modality for various cancers. In radioligand therapy, the same or a very similar radioligand as that used for imaging is coupled to a therapeutic radioisotope (e.g., Actinium-225, Lutetium-177, Yttrium-90) to deliver ionizing radiation specifically to tumors. Radioligand therapy can thus be described as a type of targeted, systemic radiotherapy. In contrast to conventional radiotherapy, radioligand therapies are systemically administered, and radioligands are internalized upon binding to tumor cells. As a consequence, metastases throughout the body can be targeted, and tumors are irradiated for hours to days.
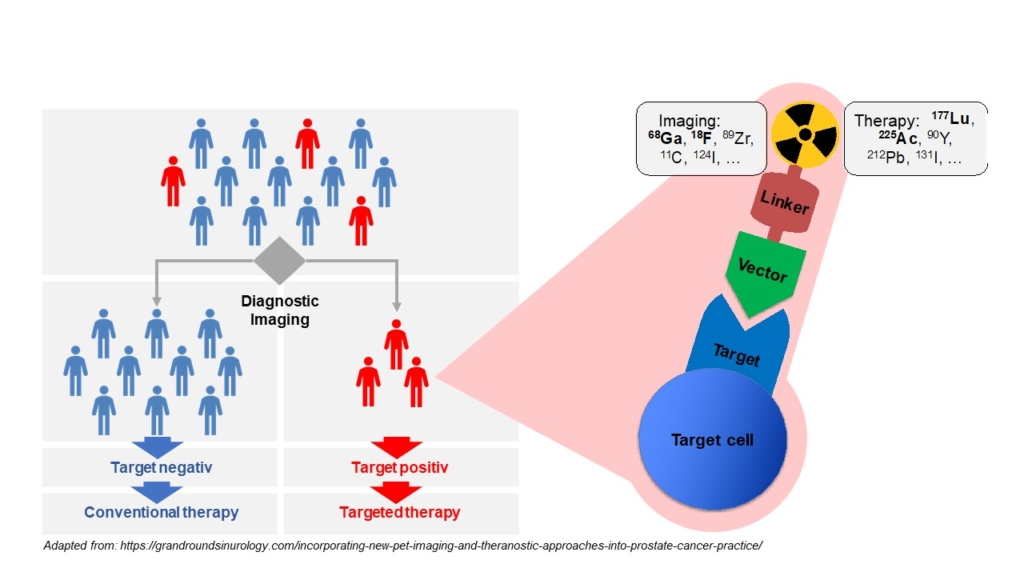
Molecular imaging in nuclear medicine combines imaging modalities like PET and SPECT with computed tomography (CT) or magnet resonance tomography to derive detailed information on disease. In the clinic, molecular imaging is mainly used for diagnosis, staging, monitoring response to therapy (e.g., by pre- and post-therapeutic FDG-PET/CT), and to select patients for RLT (e.g., PSMA-PET/CT). Our group aims at establishing molecular imaging as a phenotypic biomarker, i.e., to connect imaging information with the tumor biology, metabolism and intra- and inter-individual tumor heterogeneity. Ultimately, this will provide imaging strategies that can guide selection of individualized – and therefore more effective and less toxic – treatment regimens that improve quality of life and overall survival of patients.
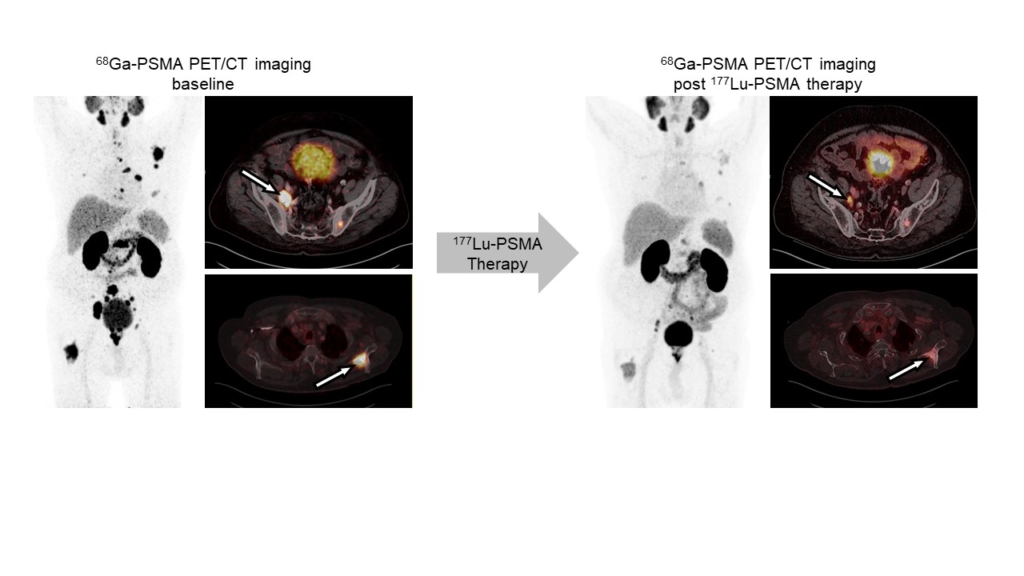
Radioligand therapy is a promising yet rarely curative oncological treatment option. Our research aims at identifying predictors (biomarkers) for and determinants (molecular and cellular processes) of response/non-response to radioligand therapy. We investigate tumor cell intrinsic resistance mechanisms, tumor protection by microenvironmental factors (immunosuppression, stroma), and suboptimal tumor radiation dose delivery using cancer models and patient samples, and diverse in vitro, ex vivo and in vivo analyses. We use the knowledge gained from these studies to rationally design and provide proof-of-principle for new radioligand therapy combination regimens that can be translated into the clinic.
Building on the first success stories of theranostics, we evaluate oncological targets and develop organic chemical or radiochemical processes for the production and development of new radioligands for imaging and therapy. This includes the design, chemical synthesis, radiolabeling, and in vitro, in vivo, and ex vivo evaluation of ligands.
The role of molecular imaging technologies in detecting, evaluating, and monitoring cardiovascular disease and their treatment is expanding rapidly. Gradually replacing the conventional anatomical or physiological approaches, molecular imaging strategies using biologically targeted markers provide unique insight into pathobiological processes at molecular and cellular levels and allow for cardiovascular disease evaluation and individualized therapy. We use SPECT and PET imaging to assess myocardial perfusion to find areas of damaged heart muscle and visualize the size and location of myocardial infarction. Specific radiotracers are used to assess cardiac inflammation and fibroblast activity in different cardiac disease models.

Prof. Dr.
Katharina Lückerath
Leiterin Präklinische Theranostik
Collaborations
Imaging / Theranostics resources for collaborators
- In vivo small animal imaging: We use the Molecubes β-Cube (PET), g-Cube (SPECT) and X-Cube (CT), specifically designed for rodent imaging. The scanners offer high resolution (spatial resolution: CT, 0.05 mm, PET, 0.85 mm, SPECT, 0.5 mm) and throughput imaging. PMOD (PFUS, PBAS, PKIN, PCARD) and Vivoquant are available for data Analysis
- Ex vivo biodistribution studies (Wizard 2480)
- Autoradiography (BetaIMAGER)
Interested in imaging or theranostics?
We’re happy to discuss collaborative projects.
Team
Präklinische Theranostik

Prof. Dr.
Katharina Lückerath
Leiterin Präklinische Theranostik

Dr. rer. nat.
Verena Behnke
Biologin

Lara Breuer
Doktorandin Präklinische Forschung

Dr. rer. nat.
Douglas Howard
Biologe

Dr. rer. nat.
Yalcin Kuzay
Biologe

Dr. rer. nat.
Magdalena Staniszewska
Biologin

Jasmin Wosniack
BTA

Dr. rer. nat.
Valeska von Kiedrowski
experimentelle Radiopharmazie

Justin Dubbert M.Sc.
experimentelle Radiopharmazie

Dr. rer. nat.
Ralph Hübner
experimentelle Radiopharmazie

Dr.
Zohreh Varasteh

Atefeh Hosseini
PhD Student
- Katharina Lückerath’s bibliography.
- Valeska von Kiedrowski’s bibliography.
- Zohreh Varasteh’s bibliography.
- Meyer C, Stuparu A, Lueckerath K, Calais J, Czernin J, Slavik R, Dahlbom M. Tandem isotope therapy with 225Ac- and 177Lu-PSMA-617 in a murine model of prostate cancer. JNM, 2023 accepted for publication
- Lückerath K, Trajkovic-Arsic M, Mona CE. Fibroblast Activation Protein Inhibitor Theranostics: Preclinical Combination Treatment. PET Clin. 2023 Jul;18(3):409-418. doi: 10.1016/j.cpet.2023.02.006. Epub 2023 Mar 27. Review. PubMed PMID: 36990945.
- Hirmas N, Hamacher R, Sraieb M, Ingenwerth M, Kessler L, Pabst KM, Barbato F, Lueckerath K, Kasper S, Nader M, Schildhaus HU, Kesch C, von Tresckow B, Hanoun C, Hautzel H, Aigner C, Glas M, Stuschke M, Kümmel S, Harter P, Lugnier C, Uhl W, Niedergethmann M, Hadaschik B, Grünwald V, Siveke JT, Herrmann K, Fendler WP. Fibroblast-Activation Protein PET and Histopathology in a Single-Center Database of 324 Patients and 21 Tumor Entities. J Nucl Med. 2023 May;64(5):711-716. doi: 10.2967/jnumed.122.264689. Epub 2022 Dec 29. Review. PubMed PMID: 36581374.
- Storey CM, Altai M, Bicak M, Veach DR, Lückerath K, Adrian G, McDevitt MR, Kalidindi T, Park JE, Herrmann K, Abou D, Zedan W, Peekhaus N, Klein RJ, Damoiseaux R, Larson SM, Lilja H, Thorek D, Ulmert D.Quantitative In Vivo Imaging of the Androgen Receptor Axis Reveals Degree of Prostate Cancer Radiotherapy Response. Mol Cancer Res. 2023 Apr 1;21(4):307-315. doi: 10.1158/1541-7786.MCR-22-0736. PubMed PMID: 36608299; PubMed Central PMCID: PMC10355285.
- Klose JM, Wosniack J, Iking J, Staniszewska M, Zarrad F, Trajkovic-Arsic M, Herrmann K, Fragoso Costa P, Lueckerath K, Fendler WP. Administration routes for SSTR- / PSMA- and FAP-directed theranostic radioligands in mice. J Nucl Med. 2022 Jan 6;. doi: 10.2967/jnumed.121.263453. [Epub ahead of print] PubMed PMID: 34992151.
- Ferdinandus J, Fendler WP, Lueckerath K, Berliner C, Kurzidem S, Hadaschik E, Klode J, Zimmer L, Livingstone E, Schadendorf D, Herrmann K, Becker JC, Ugurel S. Response to combined peptide receptor radionuclide therapy and checkpoint immunotherapy with ipilimumab plus nivolumab in metastatic Merkel cell carcinoma. J Nucl Med. 2021 Sep 2;. doi: 10.2967/jnumed.121.262344. [Epub ahead of print] PubMed PMID: 34475234.
- Staniszewska M, Fragoso Costa P, Eiber M, Klose JM, Wosniack J, Reis H, Szarvas T, Hadaschik B, Lückerath K, Herrmann K, Fendler WP, Iking J. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. Int J Mol Sci. 2021 Jul 11;22(14). doi: 10.3390/ijms22147431. PubMed PMID: 34299051; PubMed Central PMCID: PMC8304389
- Sahm A, Platzer M, Koch P, Henning Y, Bens M, Groth M, Burda H, Begall S, Ting S, Goetz M, Van Daele P, Staniszewska M, Klose JM, Costa PF, Hoffmann S, Szafranski K, Dammann P. Increased longevity due to sexual activity in mole-rats is associated with transcriptional changes in the HPA stress axis. Elife. 2021 Mar 16;10:e57843. doi: 10.7554/eLife.57843. PMID: 33724179; PMCID: PMC8012063.
- Veach DR, Storey CM, Lückerath K, Braun K, von Bodman C, Lamminmäki U, Kalidindi T, Strand SE, Strand J, Altai M, Damoiseaux R, Zanzonico P, Benabdallah N, Pankov D, Scher HI, Scardino P, Larson SM, Lilja H, McDevitt MR, Thorek DLJ, Ulmert D. PSA-Targeted Alpha-, Beta-, and Positron-Emitting Immunotheranostics in Murine Prostate Cancer Models and Nonhuman Primates. Clin Cancer Res. 2021 Apr 1;27(7):2050-2060. doi: 10.1158/1078-0432.CCR-20-3614. Epub 2021 Jan 13. PubMed PMID: 33441295.
- Czernin J, Current K, Mona CE, Nyiranshuti L, Hikmat F, Radu CG, Lückerath K. Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J Nucl Med. 2021 Feb;62(2):228-231. doi: 10.2967/jnumed.120.246041. Epub 2020 Jul 9. PubMed PMID: 32646877.
- Stuparu AD, Capri JR, Meyer C, Le TM, Evans-Axelsson SL, Current K, Lennox M, Mona CE, Fendler WP, Calais J, Eiber M, Dahlbom M, Czernin J, Radu CG, Lückerath K, Slavik R. Mechanisms of Resistance to Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in a Mouse Model of Prostate Cancer. J Nucl Med. 2020 Dec 4;. doi: 10.2967/jnumed.120.256263. [Epub ahead of print] PubMed PMID: 33277393.
- Bicak M, Lückerath K, Kalidindi T, Phelps ME, Strand SE, Morris MJ, Radu CG, Damoiseaux R, Peltola MT, Peekhaus N, Ho A, Veach D, Malmborg Hager AC, Larson SM, Lilja H, McDevitt MR, Klein RJ, Ulmert D. Genetic signature of prostate cancer mouse models resistant to optimized hK2 targeted α-particle therapy. Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):15172-15181. doi: 10.1073/pnas.1918744117. Epub 2020 Jun 12. PubMed PMID: 32532924; PubMed Central PMCID: PMC7334567.
- Kollenda SA, Klose J, Knuschke T, Sokolova V, Schmitz J, Staniszewska M, Costa PF, Herrmann K, Westendorf AM, Fendler WP, Epple M. In vivo biodistribution of calcium phosphate nanoparticles after intravascular, intramuscular, intratumoral, and soft tissue administration in mice investigated by small animal PET/CT. Acta Biomater. 2020 Jun;109:244-253. doi:10.1016/j.actbio.2020.03.031. Epub 2020 Apr 4. PMID: 32251787.
- Current K, Meyer C, Magyar CE, Mona CE, Almajano J, Slavik R, Stuparu AD, Cheng C, Dawson DW, Radu CG, Czernin J, Lueckerath K. Investigating PSMA-Targeted Radioligand Therapy Efficacy as a Function of Cellular PSMA Levels and Intratumoral PSMA Heterogeneity. Clin Cancer Res. 2020 Jun 15;26(12):2946-2955. doi: 10.1158/1078-0432.CCR-19-1485. Epub 2020 Jan 13. PubMed PMID: 31932492; PubMed Central PMCID: PMC7299755.
- Stuparu AD, Meyer CAL, Evans-Axelsson SL, Lückerath K, Wei LH, Kim W, Poddar S, Mona CE, Dahlbom M, Girgis MD, Radu CG, Czernin J, Slavik R. Targeted alpha therapy in a systemic mouse model of prostate cancer – a feasibility study. Theranostics. 2020;10(6):2612-2620. doi: 10.7150/thno.42228. eCollection 2020. PubMed PMID: 32194823; PubMed Central PMCID: PMC7052903.
- Lückerath K, Wei L, Fendler WP, Evans-Axelsson S, Stuparu AD, Slavik R, Mona CE, Calais J, Rettig M, Reiter RE, Herrmann K, Radu CG, Czernin J, Eiber M. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res.2018 Oct 29;8(1):96. doi: 10.1186/s13550-018-0451-z. PubMed PMID: 30374743; PubMed Central PMCID: PMC6206308.
- Lückerath K, Stuparu AD, Wei L, Kim W, Radu CG, Mona CE, Calais J, Rettig M, Reiter RE, Czernin J, Slavik R, Herrmann K, Eiber M, Fendler WP. Detection Threshold and Reproducibility of 68Ga-PSMA11 PET/CT in a Mouse Model of Prostate Cancer. J Nucl Med. 2018 Sep;59(9):1392-1397. doi: 10.2967/jnumed.118.207704. Epub 2018 Mar 30. PubMed PMID: 29602819; PubMed Central PMCID: PMC6910618.
- Fendler WP, Stuparu AD, Evans-Axelsson S, Lückerath K, Wei L, Kim W, Poddar S, Said J, Radu CG, Eiber M, Czernin J, Slavik R, Herrmann K. Establishing 177Lu-PSMA-617 Radioligand Therapy in a Syngeneic Model of Murine Prostate Cancer. J Nucl Med. 2017 Nov;58(11):1786-1792. doi: 10.2967/jnumed.117.193359. Epub 2017 May 25. PubMed PMID: 28546332; PubMed Central PMCID: PMC6944167.
